Abstract
Background : In the clinic, the prognostication for the survival of MDS patients is primarily determined using the IPSS and IPSS-R which are based on disease-related characteristics. Our group has previously demonstrated that the Charlson comorbidity index (CCI) and the Rockwood 9-point clinical frailty scale (CFS) are independently prognostic for overall survival (OS) in MDS patients. The CFS was used dichotomously since only 25% of patients fell within the vulnerable or frail categories. The frailty index (FI) is a popular method for measuring frailty in geriatrics and is strongly associated with the risk of adverse outcomes including death. The FI uses an accumulation of deficits approach based on the principle that the more deficits an individual has, the greater their frailty is. An individual's FI score is expressed as a ratio of deficits present to total number of deficits considered with a recommended consideration of 30 deficits.
Objective : To develop an MDS specific FI using laboratory and patient-related factors collected at baseline in a prospective national MDS registry and assess its ability to add prognostic value to the IPSS/IPSS-R.
Methods : The FI was constructed according to Searle et al.'s criteria using baseline measurements of physical performance, comorbidities, laboratory values, instrumental activities of daily living using the Lawton Brody scale, quality of life using the EQ-5D and ECOG. Factors that correlated best with age and survival were included, and FI scores were calculated only for patients who were missing <20% of the deficits considered. Univariate and multivariable Cox proportional hazard models of OS since enrollment were conducted to determine significant predictors and to generate a final model. The variables considered included age, FI score, LDH, transfusion dependence, IPSS, IPSS-R, and comorbidity using the CCI and the MDS-CI developed by Della Porta et al. Univariate Cox proportional hazard model was also performed to determine the optimal FI cutoff scores related to OS.
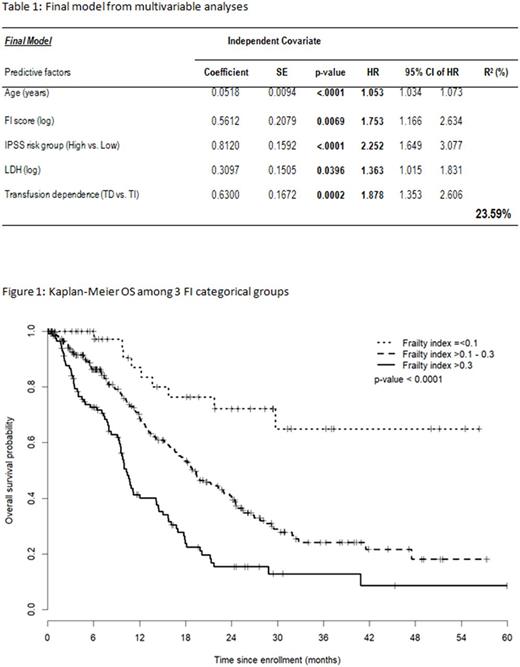
Results: The MDS-FI is comprised of 33 deficits involving 10 laboratory tests, 2 physical performance tests (4 M walk and 10 x chair sit), body mass index, 6 comorbidities, 7 Lawton Brody SIADL elements, global fatigue scale, EQ5-D elements, and ECOG. 484 MDS patients were eligible. Median age at enrollment was 73 y, 64% were male and the IPSS scores were Low (33%), INT-1 (38%), INT-2 (21%) and High (8%). Thirty-six percent of patients were transfusion dependent at enrollment. Median CCI and MDS-CI scores were 1 and 0 respectively. Actuarial survival was 16.6 months (95% CI: 14.4-18.5). The median FI score was 0.27 (IQR: 0.20-0.36) and the mean FI score 0.29 (SD=0.13). FI scores increased significantly according to IPSS risk groups whether defined in 2 or 4 risk categories (p=0.0003 and p=0.0007), and according to IPSS-R risk groups defined in either 3 or 5 risk categories (p<0.0001). The FI correlated significantly with the CFS (r=0.60, p<0.0001). Univariate analysis revealed age, FI score, LDH, transfusion dependence, IPSS (High vs. Low), IPSS-R (3 categories), CCI, and MDS-CI were significant covariates associated with OS. The multivariable model with the highest R2 value (24%) included age (p<0.0001), FI score (p=0.0069), IPSS risk (High vs. Low, p<0.0001), LDH (p=0.0396) and transfusion dependence (p=0.0002) as independent covariates best associated with OS (table 1). FI cutoff points of ≤0.1, >0.1-0.3, >0.3 had the highest R2 value of 9.28. Patients with FI > 0.3 had a higher risk of death compared to those with FI ≤ 0.1 (HR=6.50) or with FI > 0.1-0.3 (HR=2.00); patients with FI >0.1-0.3 had a higher risk of death compared to those with FI ≤ 0.1 (HR=3.25) (figure 1: p<0.0001).
Conclusions: We validated the independent influence of frailty on overall survival in MDS patients using a novel 33 deficit-based frailty index for this patient population that can be readily deployed in the clinic using available data. The additive effects of frailty on prognosis matches that of the IPSS and IPSS-R reinforcing the importance of considering both seed and soil.
Wells: Novartis: Honoraria, Membership on an entity's Board of Directors or advisory committees; Celgene: Honoraria, Membership on an entity's Board of Directors or advisory committees; Alexion: Honoraria, Membership on an entity's Board of Directors or advisory committees. Rockwood: Capital Health Research Fund: Research Funding; Fountain Family Innovation Fund of the Nova Scotia Health Authority Foundation: Research Funding; Nova Scotia Health Research Foundation: Research Funding; Dalhousie Medical Research Foundation: Research Funding; Sanofi: Research Funding; Pfizer: Research Funding; Alzheimer Society of Canada: Research Funding; Canadian Institute of Health Research: Research Funding; Lundbeck: Membership on an entity's Board of Directors or advisory committees; Research Executive Committee of the Canadian Consortium on Neurodegeneration in Aging: Membership on an entity's Board of Directors or advisory committees. Geddes: Celgene: Membership on an entity's Board of Directors or advisory committees, Research Funding; Alexion: Membership on an entity's Board of Directors or advisory committees; Novartis: Membership on an entity's Board of Directors or advisory committees. Zhu: Novartis: Consultancy; Celgene: Consultancy, Research Funding; Janssen: Consultancy. Sabloff: Celgene: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding. Keating: Bayer: Honoraria, Membership on an entity's Board of Directors or advisory committees. Leber: Celgene Canada: Honoraria, Membership on an entity's Board of Directors or advisory committees; Novartis Canada: Honoraria, Membership on an entity's Board of Directors or advisory committees. Leitch: Abbvie: Research Funding; Exjade: Speakers Bureau; Novartis: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Celgene: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Alexion: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding. Yee: Karyopharm: Research Funding; Oncoethix: Research Funding; Celgene Canada: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Astex: Research Funding; Novartis Canada: Honoraria. St-Hilaire: Novartis: Membership on an entity's Board of Directors or advisory committees; Celgene: Honoraria, Membership on an entity's Board of Directors or advisory committees. Storring: Novartis: Honoraria; Celgene: Honoraria, Research Funding. Shamy: Novartis: Membership on an entity's Board of Directors or advisory committees; Celgene: Membership on an entity's Board of Directors or advisory committees; Amgen: Membership on an entity's Board of Directors or advisory committees. Kumar: Celgene: Honoraria, Membership on an entity's Board of Directors or advisory committees. Elemary: Amgen: Membership on an entity's Board of Directors or advisory committees; Roche: Membership on an entity's Board of Directors or advisory committees; Celgene: Membership on an entity's Board of Directors or advisory committees, Research Funding; Lundbeck: Membership on an entity's Board of Directors or advisory committees; Novartis: Research Funding. Delage: Novartis: Membership on an entity's Board of Directors or advisory committees, Research Funding; Roche: Membership on an entity's Board of Directors or advisory committees, Research Funding; Celgene: Membership on an entity's Board of Directors or advisory committees; AbbVie: Research Funding; Pfizer: Research Funding; BMS: Research Funding. Buckstein: Celgene: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Novartis: Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal